Project 4: Epigenetics Impact and Testicular Cancer
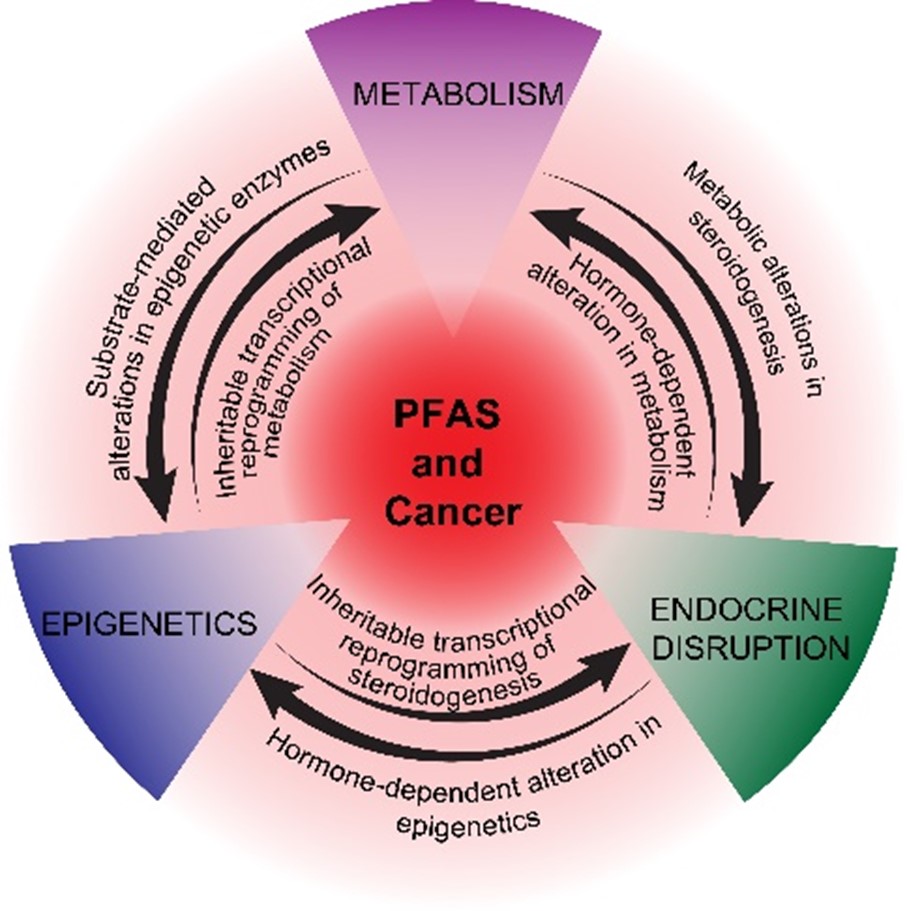
Although there is a persistent link between PFAS exposure and cancer, mechanisms are poorly understood. PFAS are non-mutagenic. Hence it is logical that epigenetic mechanisms may be key drivers of increased cancer risk upon exposure to PFAS. The best epidemiologic evidence linking PFAS to cancer is the link to testicular germ cell tumors (TGCTs). The known etiology of TGCTs strongly suggests that compared to most other solid tumors, epigenetics play an especially prominent role. We hypothesize that TGCTs may be a sentinel disease of PFAS exposure (and other non-mutagenic environmental toxicants) and that epigenetic mechanisms discovered in TGCTs may also be driving other cancers, including prostate cancer. Significance: We contend that TGCTs can be a “canary cancer in a coal mine” with rising incidence in a community indicative of unappreciated environmental toxicant exposure and furthermore, TGCTs may be an especially sensitive model to finally understand mechanisms of how PFAS and other non-mutagenic environmental toxicants drive cancer. Testicular cancer is an aggressive disease that inflicts men in the prime of life and results in the highest number of life years lost per death of any adult cancer. We have recently discovered using de novo “omic” approaches that the polycomb pathway and DNA methylation are interconnected epigenetic drivers of cisplatin and epigenetic therapy sensitivity and tumorigenicity of TGCTs. Excitingly, we have shown that PFAS strongly induce the polycomb pathway in normal mouse testis and also in TGCTs leading to alterations in tumorigenicity. The hypothesis of the current proposal is that PFAS promote and alter testicular cancer progression and response to therapy through altering polycomb and other co-dependent epigenetic, metabolic and endocrine disrupting pathways. To test this hypothesis, we have developed unique model systems including cisplatin and epigenetic therapy resistant TGCT cells and cells with distinct genetically engineered perturbations in the polycomb and DNA methylation pathways. Our approach will use cell models and xenografts to uncover detailed mechanisms of how PFAS alter the epigenetic state of TGCT cells and will also employ animal models including a state of the art GEM model to uncover whether PFAS have similar effects on male germ cells in vivo and also whether PFAS can promote the initiation of TGCTs via these same mechanisms. Finally, mechanisms discovered will be assessed in human clinical specimens. We are uniquely situated to pursue this exciting epigenetic-based convergence of PFAS exposure and TGCT progression. SRP Mandates: Our work will define PFAS mediate epigenetic dysregulation and pro-cancer phenotypes during various acute/chronic developmental windows in a spontaneous model of TGCTs (SRP#2). Our studies will identify early biomarkers of PFAS exposure and provide mechanistic insight as to how PFAS exposure promotes testicular cancer aggressiveness and alters response to chemotherapy and emerging epigenetic therapies (SRP#1,4). This research addresses the broader scope of E-PACT to address cancer effects of PFAS with targeted community outreach.
Select publications:
Boyd, R., Shokry, D., Fazal, Z., Rennels, B., Freemantle, S., La Frano, M., Prins, G., Madak Erdogan, Z., Irudayaraj, J., Singh, R., Spinella, M. Perfluorooctanesulfonic acid alters pro-cancer phenotypes and metabolic and transcriptional signatures in testicular germ cell tumors. 2024. Toxics, 12(4), 232. https://doi.org/10.3390/toxics12040232
Sands, M., Zhang, X., Spinella, M., Madak-Erdogan, Z., and Irudayaraj, J. Comparative Hepatotoxicity of Novel Lithium Bis(trifluoromethanesulfonyl)imide (LiTFSI) and Legacy Perfluorooctanoic Acid (PFOA) in Male Mice: Insights into Epigenetic Mechanisms and Pathway-Specific Responses. 2024. Environment International, 108556. https://doi.org/10.1016/j.envint.2024.108556
Zhang, X., Sands, M., La Frano, M., Spinella, M., Masoud, F., Fields, C., Madak-Erdogan, Z., Jensen, T., and Irudayaraj, J. MicroRNA’s and PFAS: A pilot study in blood collected from firefighters. 2024. bioRXiv: doi: https://doi.org/10.1101/2024.04.05.588341.
2023
Zhang X, Flaws JA, Spinella MJ, Irudayaraj J. The Relationship between Typical Environmental Endocrine Disruptors and Kidney Disease. Toxics. 2023;11(1):32. PubMed PMID: doi:10.3390/toxics11010032.
2022
Wen Y, Rashid F, Fazal Z, Singh R, Spinella MJ, Irudayaraj J. Nephrotoxicity of perfluorooctane sulfonate (PFOS)-effect on transcription and epigenetic factors. Environ Epigenet. 2022;8(1):dvac010. Epub 2022/06/01. doi: 10.1093/eep/dvac010. PubMed PMID: 35633893; PMCID: PMC9134076.
Singh R, Fazal Z, Bikorimana E, Boyd RI, Yerby C, Tomlin M, Baldwin H, Shokry D, Corbet AK, Shahid K, Hattab A, Freemantle SJ, Spinella MJ. Reciprocal epigenetic remodeling controls testicular cancer hypersensitivity to hypomethylating agents and chemotherapy. Molecular Oncology. 2022;16(3):683-98. doi: https://doi.org/10.1002/1878-0261.13096.
Boyd RI, Ahmad S, Singh R, Fazal Z, Prins GS, Madak Erdogan Z, Irudayaraj J, Spinella MJ. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers (Basel). 2022;14(12). Epub 2022/06/25. doi: 10.3390/cancers14122919. PubMed PMID: 35740585; PMCID: PMC9220899.
2021
Singh R, Fazal Z, Freemantle SJ, Spinella MJ. Between a Rock and a Hard Place: An Epigenetic-Centric View of Testicular Germ Cell Tumors. Cancers. 2021;13(7):1506. PubMed PMID: doi:10.3390/cancers13071506.
Singh R, Bikorimana E, Fazal Z, Yerby C, Baldwin H, Hattab AD, Tomlin M, Corbet AK, Boyd RI, Shahid K, Shokry DNAM, Spinella SJ, Spinella MJ. Abstract 2127: Polycomb signaling reciprocally regulates sensitivity of testicular germ cell tumors cells to cisplatin and DNA hypomethylating agents. Cancer Research. 2021;81(13_Supplement):2127-. doi: 10.1158/1538-7445.Am2021-2127.
Imir OB, Kaminsky AZ, Zuo QY, Liu YJ, Singh R, Spinella MJ, Irudayaraj J, Hu WY, Prins GS, Madak Erdogan Z. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients. 2021;13(11). Epub 2021/11/28. doi: 10.3390/nu13113902. PubMed PMID: 34836157; PMCID: PMC8623692.
Fazal Z, Singh R, Tomlin M, Shokry D, Hattab A, Spinella S, Spinella M. Abstract 2096: Genome wide remodeling of DNA methylation and CpG hypermethylation is tightly linked to acquired resistance in testicular cancer. Cancer Research. 2021;81(13_Supplement):2096-. doi: 10.1158/1538-7445.Am2021-2096.
Fazal Z, Singh R, Fang F, Bikorimana E, Baldwin H, Corbet A, Tomlin M, Yerby C, Adra N, Albany C, Lee S, Freemantle SJ, Nephew KP, Christensen BC, Spinella MJ. Hypermethylation and global remodelling of DNA methylation is associated with acquired cisplatin resistance in testicular germ cell tumours. Epigenetics. 2021;16(10):1071-84. doi: 10.1080/15592294.2020.1834926.
Albany C, Fazal Z, Singh R, Bikorimana E, Adra N, Hanna NH, Einhorn LH, Perkins SM, Sandusky GE, Christensen BC, Keer H, Fang F, Nephew KP, Spinella MJ. A phase 1 study of combined guadecitabine and cisplatin in platinum refractory germ cell cancer. Cancer Medicine. 2021;10(1):156-63. doi: https://doi.org/10.1002/cam4.3583.
2019
Singh R, Fazal Z, Freemantle SJ, Spinella MJ. Mechanisms of cisplatin sensitivity and resistance in testicular germ cell tumors. Cancer Drug Resist. 2019;2(3):580-94. Epub 2019/09/21. doi: 10.20517/cdr.2019.19. PubMed PMID: 31538140; PMCID: PMC6752046.
Singh R, Fazal Z, Corbet AK, Bikorimana E, Rodriguez JC, Khan EM, Shahid K, Freemantle SJ, Spinella MJ. Epigenetic Remodeling through Downregulation of Polycomb Repressive Complex 2 Mediates Chemotherapy Resistance in Testicular Germ Cell Tumors. Cancers. 2019;11(6):796. PubMed PMID: doi:10.3390/cancers11060796.
