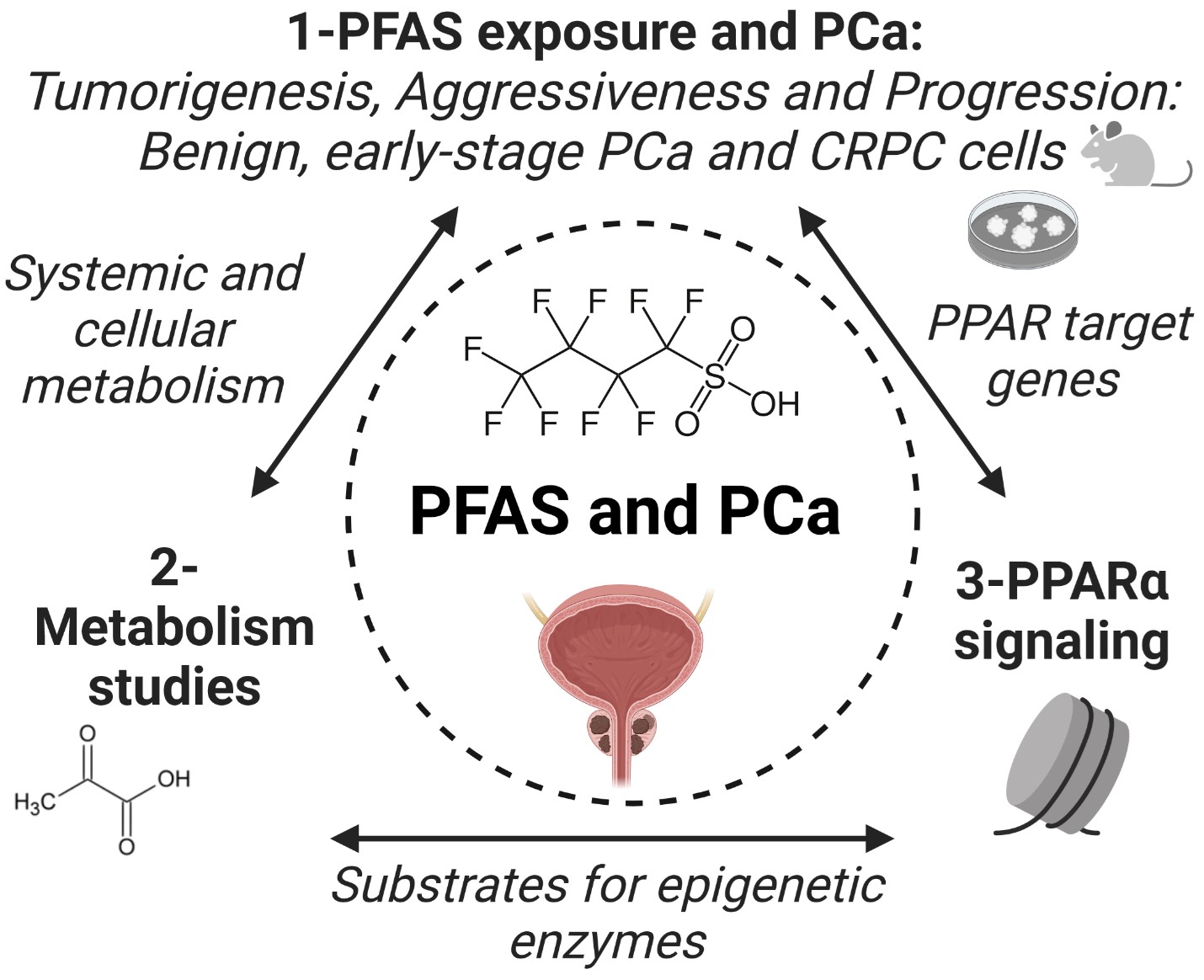
Project 3: PFAS as Drivers of Prostate Carcinogenesis and Progression
Environmental exposures contribute to risk and progression of prostate cancer (PCa), the most prevalent non-cutaneous cancer in American men. Several epidemiology studies have linked PFAS exposures in occupational and contamination settings to increased PCa susceptibility and mortality. Whether chronic PFAS exposures at lower doses may also contribute to increased PCa risk and aggression is presently unknown and potential mechanisms that may be at play are unexplored. The overall objective of Project 3 is to elucidate the actions of PFAS that drive prostate carcinogenesis and promote metastatic growth leading to increased PCa mortality.
We hypothesize that PFAS exposures drive metabolic changes that reprogram prostate cells through epigenetic modifications that, independently or combined, initiate PCa and promote tumor progression including metastatic expansion. We will test this hypothesis with advanced methodologies using clinically relevant models in three Specific Aims. Aim 1: Delineate the impact of PFAS on prostate tumorigenicity, aggressiveness and metastatic growth. Disease-free, patient-derived human prostate epithelial cells (PrECs), benign prostate cell lines, PCa cell lines that model early-stage PCa (RWPE-kRAS, RWPE-AKT1, RWPE-cMYC) and metastatic human-derived CWR-R1 cells and PDX models will be used in a rigorous set of in vitro and in vivo assays to comprehensively assess of the carcinogenic potential, tumor-promoting activity and metastatic enhancement of PFAS chemicals in the prostate. AIM 2: Define the metabolic signature of PFAS-exposed benign and cancerous prostate cells and tissues. Metabolomics and transcriptomics analyses with small molecule inhibitors and knockdown approaches will be used to define and characterize a novel PFAS-induced onco-metabolome that drives PCa growth and progression. AIM 3: Determine how PFAS impacts the epigenome PPARα signaling. Studies will interrogate how PFAS reprograms epigenetic marks that dictate the PPARα cistrome and transcriptome to activate pathways that ensure prostate cell transformation, PCa survival and metastatic expansion.
Sands, M., Zhang, X., Spinella, M., Madak-Erdogan, Z., and Irudayaraj, J. Comparative Hepatotoxicity of Novel Lithium Bis(trifluoromethanesulfonyl)imide (LiTFSI) and Legacy Perfluorooctanoic Acid (PFOA) in Male Mice: Insights into Epigenetic Mechanisms and Pathway-Specific Responses. 2024. Environment International, 108556. https://doi.org/10.1016/j.envint.2024.108556
Boyd, R., Shokry, D., Fazal, Z., Rennels, B., Freemantle, S., La Frano, M., Prins, G., Madak Erdogan, Z., Irudayaraj, J., Singh, R., Spinella, M. Perfluorooctanesulfonic acid alters pro-cancer phenotypes and metabolic and transcriptional signatures in testicular germ cell tumors. 2024. Toxics, 12(4), 232. https://doi.org/10.3390/toxics12040232
Zhang, X., Sands, M., La Frano, M., Spinella, M., Masoud, F., Fields, C., Madak-Erdogan, Z., Jensen, T., and Irudayaraj, J. MicroRNA’s and PFAS: A pilot study in blood collected from firefighters. 2024. bioRXiv: doi: https://doi.org/10.1101/2024.04.05.588341.
2023
Bosland MC, Schlicht MJ, Acevedo N, Soto AM, Prins G. Effects of perinatal exposure to bisphenol A on induction of prostate cancer in Sprague Dawley rats by MNU and testosterone. Toxicology. 2023;484:153394. doi: https://doi.org/10.1016/j.tox.2022.153394.
Garcia J, Krieger KD, Loitz C, Perez LM, Richards ZA, Helou Y, Kregel S, Celada S, Mesaros CA, Bosland M, Gann PH, Willnow TE, Vander Griend D, Kittles R, Prins GS, Penning T, Nonn L. Regulation of Prostate Androgens by Megalin and 25-hydroxyvitamin D Status: Mechanism for High Prostate Androgens in African American Men. Cancer Research Communications. 2023;3(3):371-82. doi: 10.1158/2767-9764.Crc-22-0362.
Vandenberg LN, Zoeller RT, Prins GS, Trasande L. Evaluating adverse effects of environmental agents in food: a brief critique of the US FDA’s criteria. Environmental Health. 2023;22(1):38. doi: 10.1186/s12940-023-00971-2.
Santaliz-Casiano A, Mehta D, Danciu OC, Patel H, Banks L, Zaidi A, Buckley J, Rauscher GH, Schulte L, Weller LR, Taiym D, Liko-Hazizi E, Pulliam N, Friedewald SM, Khan S, Kim JJ, Gradishar W, Hegerty S, Frasor J, Hoskins KF, Madak-Erdogan Z. Identification of metabolic pathways contributing to ER+ breast cancer disparities using a machine-learning pipeline. Sci Rep. 2023 Jul 26;13(1):12136. doi: 10.1038/s41598-023-39215-1. PMID: 37495653; PMCID: PMC10372029.
Mogol, A. N., Zuo, Q., Yoo, J. Y., Kaminsky, A. Z., Imir, O. B., Landesman, Y., Walker, C. J., & Madak Erdogan, Z. (2023). NAD + Metabolism Generates a Metabolic Vulnerability in Endocrine-Resistant Metastatic Breast Tumors in Females. Endocrinology, bqad073. https://doi.org/10.1210/endocr/bqad073
2022
Hu W-Y, Lu R, Hu DP, Imir OB, Zuo Q, Moline D, Afradiasbagharani P, Liu L, Lowe S, Birch L, Griend DJV, Madak-Erdogan Z, Prins GS. Per- and polyfluoroalkyl substances target and alter human prostate stem-progenitor cells. Biochemical Pharmacology. 2022;197:114902. doi: https://doi.org/10.1016/j.bcp.2021.114902.
Santaliz Casiano A, Lee A, Teteh D, Madak Erdogan Z, Treviño L. Endocrine-Disrupting Chemicals and Breast Cancer: Disparities in Exposure and Importance of Research Inclusivity. Endocrinology. 2022;163(5). Epub 2022/03/25. doi: 10.1210/endocr/bqac034. PubMed PMID: 35325096; PMCID: PMC9391683.
Hu WY, Liu LF, Afradiasbagharani P, Lu RL, Chen ZL, Hu DP, Birch LA, Prins GS. Stem cells from a malignant rat prostate cell line generate prostate cancers in vivo: a model for prostate cancer stem cell propagated tumor growth. Am J Clin Exp Urol. 2022;10(6):377-89. Epub 2023/01/14. PubMed PMID: 36636689; PMCID: PMC9831920.
Boyd RI, Ahmad S, Singh R, Fazal Z, Prins GS, Madak Erdogan Z, Irudayaraj J, Spinella MJ. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers (Basel). 2022;14(12). Epub 2022/06/25. doi: 10.3390/cancers14122919. PubMed PMID: 35740585; PMCID: PMC9220899.
Mitra C, Yoo JY, Madak-Erdogan Z, Soliman A, editors. Spatial Analysis of Tumor Heterogeneity Using Machine Learning Techniques. 2022 IEEE 19th International Conference on Mobile Ad Hoc and Smart Systems (MASS); 2022 19-23 Oct. 2022.
2021
Imir OB, Kaminsky AZ, Zuo QY, Liu YJ, Singh R, Spinella MJ, Irudayaraj J, Hu WY, Prins GS, Madak Erdogan Z. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients. 2021;13(11). Epub 2021/11/28. doi: 10.3390/nu13113902. PubMed PMID: 34836157; PMCID: PMC8623692.
Hu W-Y, Hu D-P, Xie L, Nonn L, Lu R, Abern M, Shioda T, Prins GS. Keratin Profiling by Single-Cell RNA-Sequencing Identifies Human Prostate Stem Cell Lineage Hierarchy and Cancer Stem-Like Cells. International Journal of Molecular Sciences. 2021;22(15):8109. PubMed PMID: doi:10.3390/ijms22158109.
Hu W-Y, Afradiasbagharani P, Lu R, Liu L, Birch LA, Prins GS. Morphometric Analysis of Rat Prostate Development: Roles of MEK/ERK and Rho Signaling Pathways in Prostatic Morphogenesis. Biomolecules. 2021;11(12):1829. PubMed PMID: doi:10.3390/biom11121829.
2020
Prins GS, editor. Role of endocrine disruptors in cancer development. CANCER PREVENTION RESEARCH; 2020: AMER ASSOC CANCER RESEARCH 615 CHESTNUT ST, 17TH FLOOR, PHILADELPHIA, PA ….

